Molybdenum Stock
Stock Supplies
These sizes are in stock, subject to prior sale. We also supply other sizes and alloys as well. See additional material information below.
Size to Weight Calculatorsheet
- .001″ x 12″ Coil
- .002″ x 12″ Coil
- .003″ x 12″ x Coil
- .005″ x 12″ x 36″
- .005″ x 24″ x Coil
- .010″ x 24″ x Coil
- .015″ x 24″ x Coil
- .020″ x 24″ x Coil
- .025″ x 24″ x R/L
- .030″ x 24″ x R/L
- .040″ x 24″ x R/L
- .060″ x 24″ x R/L
- .080″ x 12″ x R/L
- .090″ x 12″ x R/L
- .125″ x 12″ x R/L
- .187″ x 12″ x R/L
plate
- .250″ x 12″ x R/L
- .280″ x 24″ x R/L
- .375″ x 12″ x R/L
- .500″ x 12″ x R/L
- .625″ x 12″ x R/L
- .750″ x 12″ x R/L
- 1.0″ x 12″ x R/L
- 1.125″ x 12″ x R/L
- 1.187″ x 12″ x R/L
- 1.250″ x 12″ x R/L
- 1.375″ x 12″ x R/L
- 1.500″ x 12″ x R/L
- 1.750″ x 12″ x R/L
- 2.0″ x 12″ x R/L
wire
- .005″ Dia x 200 ft
- .010″ Dia x 200 ft
- .015″ Dia x 200 ft
- .020″ Dia x 200 ft
- .025″ Dia x 200 ft
- .030″ Dia x 200 ft
- .050″ Dia x 200 ft
- .060″ Dia x R/L Coil
- .080″ Dia x R/L Coil
- .090″ Dia x R/L Coil
- .125″ Dia x R/L Coil
rod
- .040″ Dia x R/L
- .050″ Dia x R/L
- .060″ Dia x R/L
- .062″ Dia x R/L
- .080″ Dia x R/L
- .090″ Dia x R/L
- .093″ Dia x R/L
- .125″ Dia x R/L
- .156″ Dia x R/L
- .187″ Dia x R/L
- .250″ Dia x R/L
- .312″ Dia x R/L
- .375″ Dia x R/L
- .437″ Dia x R/L
- .500″ Dia x R/L
- .625″ Dia x R/L
- .750″ Dia x R/L
- .875″ Dia x R/L
- 1.000″ Dia x R/L
- 1.125″ Dia x R/L
- 1.187″ Dia x R/L
- 1.250″ Dia x R/L
- 1.375″ Dia x R/L
- 1.500″ Dia x R/L
- 1.625″ Dia x R/L
- 1.750″ Dia x R/L
- 2.000″ Dia x R/L
- 2.125″ Dia x R/L
- 2.250″ Dia x R/L
- 2.375″ Dia x R/L
- 2.500″ Dia x R/L
- 2.625″ Dia x R/L
- 2.750″ Dia x R/L
- 3.000″ Dia x R/L
- 3.125″ Dia x R/L
- 3.250″ Dia x R/L
- 3.500″ Dia x R/L
- 3.750″ Dia x R/L
- 4.000″ Dia x R/L
- 4.500″ Dia x R/L
- 5.000″ Dia x R/L
tubing
- .125″ OD x .015″ wall x R/L
- .187″ OD x .020″ wall x R/L
- .250″ OD x .020″ wall x R/L
- .312″ OD x .020″ wall x R/L
- .375″ OD x .025″ wall x R/L
- .500″ OD x .030″ wall x R/L
- .625″ OD x .050″ wall x R/L
- .750″ OD x .060″ wall x R/L
- .875″ OD x .040″ wall x R/L
- 1.0″ OD x .050″ wall x R/L
Molybdenum Facts & FAQs
Molybdenum is number 42 on the periodic table. With a melting point of 2622 °C (4752 °F), molybdenum has a density of 10.22 gm/cc. It has many properties that make it an excellent candidate for fabricated parts that must be made of a refractory metal.
Molybdenum has been used for many years in the lamp industry for mandrels and supports, usually in wire form. Today, several unique properties of molybdenum that satisfy more demanding industry requirements have increased the use of molybdenum as a material in applications requiring other mill forms.
What is Molybdenum?
What were the First Applications?
Modern Applications of Molybdenum
What is Moly Used For?
High Temperatures
Heat Treating
Glassmaking
Other Hot Working and Processing Applications
Medical Uses
What is Molybdenum?
What is Molybdenum?
Molybdenum (pronounced muh-lib-duh-nuh m), frequently called “moly,” is an element on the periodic table that was discovered in 1871 by Carl Scheele. This element is vitally important to nearly every phase of our economy, and even to our personal health. It works behind the scenes to enable technologies that have revolutionized the world.
Pure moly has a very high melting point [2622 °C (4752 °F)]. Only four metallic elements (tantalum, osmium, rhenium, and tungsten) melt at higher temperatures.
Tungsten (https://www.rembar.com/our-stock/tungsten) is the only metal whose thermal expansion coefficient is smaller than moly’s, and molybdenum’s heat capacity (https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK- 12)/17%3A_Thermochemistry/17.04%3A_Heat_Capacity_and_Specific_Heat) is only about 60% of that
of common metals like iron, nickel, and cobalt.
These seemingly random properties work together to make it an ideal material for many applications, a fact that has remained true even as technology has continued to evolve.
What were the First Applications?
Moly was first used in volume as support wires for tungsten filaments in incandescent lamps. Its high-temperature strength and compatibility with tungsten, combined with its lower processing cost, made it the perfect choice.
Technological advances led to electronic vacuum tubes, where moly served as internal components like grids and other tube structures.
Evolution in lighting led to halogen and high-intensity discharge (HID) lamps, which operate at a much higher temperature than standard lamps. They required high melting-point glass envelopes, and moly’s thermal expansion coefficient is a perfect fit for power feed-throughs bonded to the lamp envelope.
A molybdenum alloy (https://www.rembar.com/our-stock/moly-alloys/) containing dispersed fine yttrium oxide and cerium oxide particles to improve the glass bond strength has superseded the traditional pure moly foil used for this application.

Modern Applications of Molybdenum
As electronics moved from the world of vacuum tubes to solid-state devices, new applications replaced the old. Transistors create heat that must be removed to avoid early device failure. In addition, the silicon wafers from which transistors are made are quite fragile.
Moly, with its high thermal conductivity and a thermal expansion coefficient that is closely matched to silicon’s, is crucial in removing heat from and providing a strong and robust base for power transistors. Moly heat sinks are used in tiny diodes carrying minuscule amounts of current, power transistors operating at thousands of volts and amps, and devices of all sizes in between. Combined with copper as a cladding or as an intimate mixture of bonded particles, moly is used in power modules for hybrid cars and mobile phone base stations. Moly thin films are found in the circuitry of liquid-crystal display (LCD) screens and advanced solar- powered modules.
What is Moly Used For?
Moly is also found in components of the equipment that manufactures solid-state devices. Its strength and stiffness at high temperature and resistance to aggressive environments make it the choice for components in ion implantation equipment.
Ion implanters dope silicon substrates with boron, phosphorus, or arsenic to customize the electrical properties of the finished device. To do this, ion implanters create a high-temperature plasma containing the dopant ions, which are accelerated by high voltages toward a silicon substrate and driven into the silicon. The ion plasma is highly reactive and must be contained and guided by components impervious to its attack. Molybdenum is an ideal material for implanter arc chambers and other components because of its high-temperature strength and compatibility with the gases used to create the plasma. It also finds use in other equipment; for example, molecular beam epitaxy (MBE) and physical vapor deposition (PVD) equipment.
In high-temperature vacuum furnaces Moly is used for heating elements, heat shields, furnace racks, and other internal hardware. Because it is very pure and has a low vapor pressure at high temperature Moly contributes to very low impurity levels in the chamber during operation.
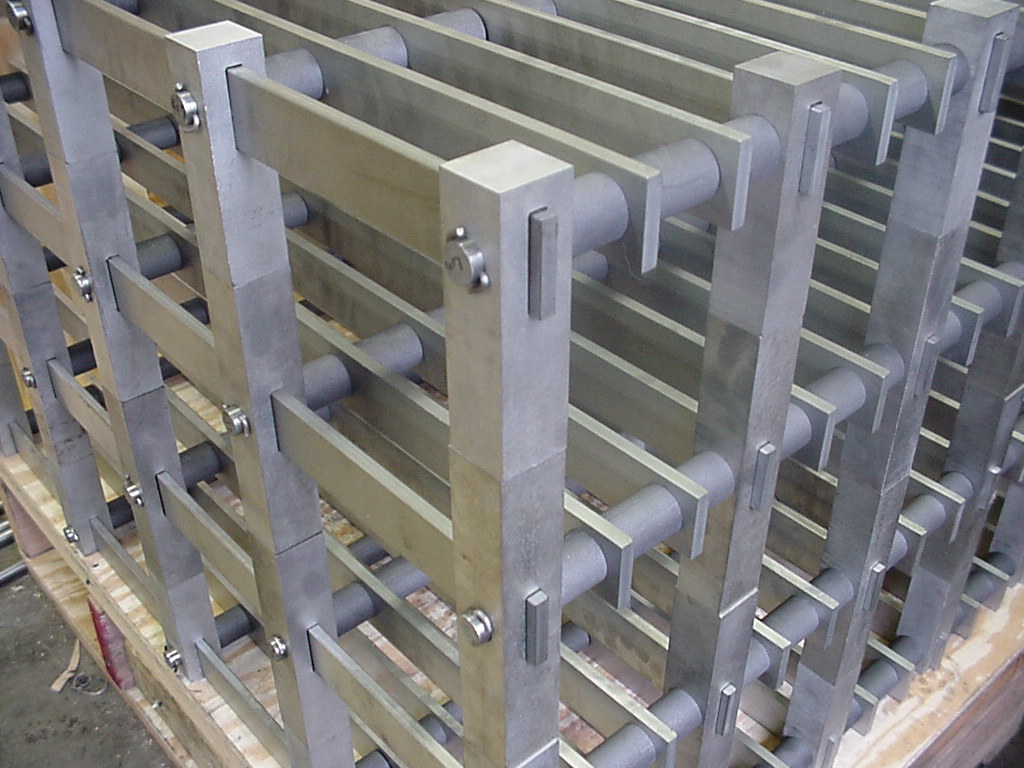
High Temperatures
Heat Treating
Molybdenum has long been an essential material for applications requiring strength at high temperatures. Moly-based vacuum furnaces are the first choice for heat treating materials that, like titanium, react with carbon to render parts unusable. For temperatures up to about 1200 °C (2190 °F), moly is the furnace material of choice.
The ultimate example of moly-lined furnaces is the hot isostatic press (HIP), which is essentially a furnace inside a pressure vessel that can apply gas pressures of 30,000 psi to the furnace load. HIP technology is an important process in the aerospace industry, where it is used to close pores and internal defects in critical parts like turbine discs. It is also becoming a mainstay in processing parts made using additive manufacturing (AM) techniques.
Molybdenum is also the perfect material for heating elements in hydrogen-atmosphere furnaces because it is impervious to attack or hydrogen absorption. Moly is not a good choice for atmospheres that contain oxygen because MoO3 evaporates at temperatures above about 600 °C (1110 °F).
Hydrogen furnaces protect molybdenum components because the hydrogen reacts with any oxygen or oxides present to keep moly heating elements and structures clean.

Glassmaking
Moly’s compatibility with most molten glasses, its high-temperature strength, and good electrical conductivity facilitated a revolution in glassmaking. Since molten glass conducts electricity, passing current through a glass bath can boost glassmaking capacity and greatly increase the throughput of traditional flame-heated glass furnaces. Molybdenum’s high-temperature strength and stability and compatibility with many glass compositions make it a perfect electrode material in electric-boost glass furnaces.
The molybdenum electrodes are often inserted into the bath from below through penetrations in the furnace refractory. This way, they are protected by the molten glass bath from oxidation. As the electrodes erode during service, they are replaced by attaching new lengths of electrode to the external end and pushing the modified electrode into the melt. This way, only the electrode being rejuvenated needs to be shut down, and only until it is repositioned. There is no need to shut down furnaces and suffer long delays for electrode replacement. Newer furnace designs also employ moly plate in areas such as walls and channels where flowing glass erodes the furnace brick.
Other Hot Working and Processing Applications
Molybdenum components process high-temperature liquids other than glass. Alloyed with tungsten, Moly is an ideal material to handle molten zinc because it resists zinc corrosion. Synthetic sapphire, the extremely scratch-resistant material often seen in bar-code scanner windows, laser windows, and armor, with a melting temperature of 2030-2050 °C (3686-3722 °F), is processed in moly crucibles.
Hot processing of solids is another area where moly excels. The most impressive of these processes is hot isothermal forging, where both the workpiece (usually a nickel-based superalloy jet engine part) and the dies themselves are heated in inert atmosphere or under vacuum to between 1830 °F and 2190 °F (1000 °C to 1200 °C). Forging pressure is then applied to both the tool and the workpiece for a controlled time.
“Superplastic” deformation during the process produces a near-net-shape forging with greatly reduced machining cost and better mechanical properties compared to traditionally forged parts. Only dies and other components made from molybdenum TZM (Mo-0.5% Ti-0.08% Zr) and MHC (Mo-1/2% Hf- 0.025% C) alloys can withstand this process.
Other hot working applications for these alloys include piercing plugs for seamless steel tubing production and dies for hot extruding brass alloys.
Medical Uses
Molybdenum components are also important in medical technology, such as:
- Traditional X-ray diagnostic equipment
- CAT scanning machines
- Cardiac stents
- Spinal implants
In a CAT scanner, pure molybdenum cathode cups focus an electron beam produced by a tungsten filament in the cathode assembly onto a target strip of tungsten-rhenium alloy embedded in a moly-TZM alloy rotating anode. The electron beam causes the tungsten alloy to emit high-energy X-rays. The anode spins at about 10,000 rpm to avoid overheating, but the tungsten track still heats to temperatures near 70% of the alloy’s melting temperature.
Moly’s high-temperature strength, low thermal expansion coefficient, high thermal conductivity and low heat capacity work together to make it an efficient material to transmit this heat to a graphite heat sink mounted on the disc (not shown in the accompanying image of an older tube design), which transfers the heat to a surrounding oil bath. At the imaging end of the scanner, thin Moly sheet is used in the collimating section that blocks stray radiation and ensures the clearest image possible.
Another medical application is a moly-rhenium alloy used to make cardiac stents and spinal implants. Rhenium, the densest material in the periodic table, increases the alloy’s strength and ductility compared to pure moly. The alloy absorbs X-rays more efficiently, improving the devices’ visibility in X-ray images used by surgeons during location and installation of stents. The alloy’s increased strength ductility, and toughness provides a margin of safety against mechanical failure.
Molybdenum Facts
Molybdenum is number 42 on the periodic table. With a melting point of 2610°C, molybdenum has a density of 10.22 gm/cc. It has many properties that make it an excellent candidate for fabricated parts that must be made of a refractory metal. Molybdenum has been used for many years in the lamp industry for mandrels and supports, usually in wire form. Today, several unique properties of molybdenum that satisfy more demanding industry requirements have increased the use of molybdenum as a material in applications requiring other mill forms.

MATERIAL INFORMATION
ASTM B386, ASTM B387, Type 361
ASTM Certifications upon request
No cutting fee for custom lengths
Custom Sizes Available
Custom Stocking Plans Available
Technical Information
Atomic Number: 42
Atomic Weight: 95.94
Density: (20°C) 10.22 g/CC
Melting Point: 2896 K, 2610°C, 4753°Fm
Boiling Point: 4912 K, 5560°C, 8382°F
Coefficient of Thermal Expansion: (20°C) 4.9 x 10-6/°C
Electrical Resistivity: (20°C) 5.7 microhms-cm
Electrical Conductivity: 30% IACS
Specific Heat: .061 cal/g/°C
Thermal Conductivity: .35 cal/cm2/cm°C/sec
Modulus of Elasticity: (20°C) 46 x 106 psi
Mass:
Density at 20° C gm/cc - 10.2
Density at 20° C lb/in 3 - 0.368
Thermal Properties:
Melting Point, °C - 2610
Boiling Point, °C - 5560
Linear Coefficient of Expansion per °C - 4.9 x 10-6
Thermal Conductivity at 20°C, cal/cm2/cm°C/sec. - 0.35
Specific Heat, cal/g/°C; 20°C - 0.061
Electrical Properties:
Conductivity, % IACS - 30%
Resistivity, microhms-cm; 20°C - 5.7
Temperature Coefficient of Resistivity per °C (0-100°C) - 0.0046
Mechanical Properties:
Tensile Strength at room temperature, psi - 100,000-200,000
Tensile Strength-500°C psi - 35,000-65,000
Tensile Strength-1000°C psi - 20,000-30,000
Young's Modulus of Elasticity; lb/in2:
Room Temperature - 46 x 106
500°C - 41 x 106
1000°C - 39 x 106
Spectral Emissivity:
(Wave Length approx. 0.65) - 0.37 (1000°C)
Working Temperature: 1600°C
Recrystallizing Temp: 900-1200°C
Stress Relieving Temp: 800°C
Metallography:
Etchant - Hot H2O2; 6% sol
Polishing - Alumina - Rouge to finish
Heat Shields
Furnace Temperature, x F - 1832, 1832, 2012, 2012, 2400, 2400
Cold Shell Temperature, x F - 100, 100, 100, 100, 100, 100
Number of Shields (1 - 10) - 1, 2, 1, 2, 1, 2
Avg. Shield Emissivity Factor (0 - 1.0) - 0.60, 0.60, 0.60, 0.60, 0.60, 0.60
Cold Shell Emissivity Factor (.9 typ) - 0.90, 0.90, 0.90, 0.90, 0.90, 0.90
Computed Shield Temperature IN x F
#1 Shield - 1484, 1646, 1637, 1811, 1965, 2167
#2 Shield - 1250, 1384, 1672
Computed Heat Loss (KW/ft2) - 4.0, 2.4, 5.5, 3.3, 9.8, 5.9
Furnace Temperature, x F - 2400, 2400, 2400, 2400, 2192, 2192
Cold Shell Temperature, x F - 150, 150, 150, 150, 100, 100
Number of Shields (1 - 10) - 3, 4, 5, 6, 1, 2
Avg. Shield Emissivity Factor (0 - 1.0) - 0.60, 0.60, 0.60, 0.60, 0.60, 0.60
Cold Shell Emissivity Factor (.9 typ) - 0.70, 0.70, 0.70, 0.70, 0.90, 0.90
Computed Shield Temperature IN x F
#1 Shield - 2247, 2282, 2305, 2320, 1789, 1976
#2 Shield - 1976, 2087, 2151, 2194, 1517
#3 Shield - 1563, 1832, 1966, 2047
#4 Shield - 1444, 1723, 1869
#5 Shield - 1354, 1636
#6 Shield - 1282
Computed Heat Loss (KW/ft2) - 4.0, 3.1, 2.6, 2.2, 7.3, 4.3
